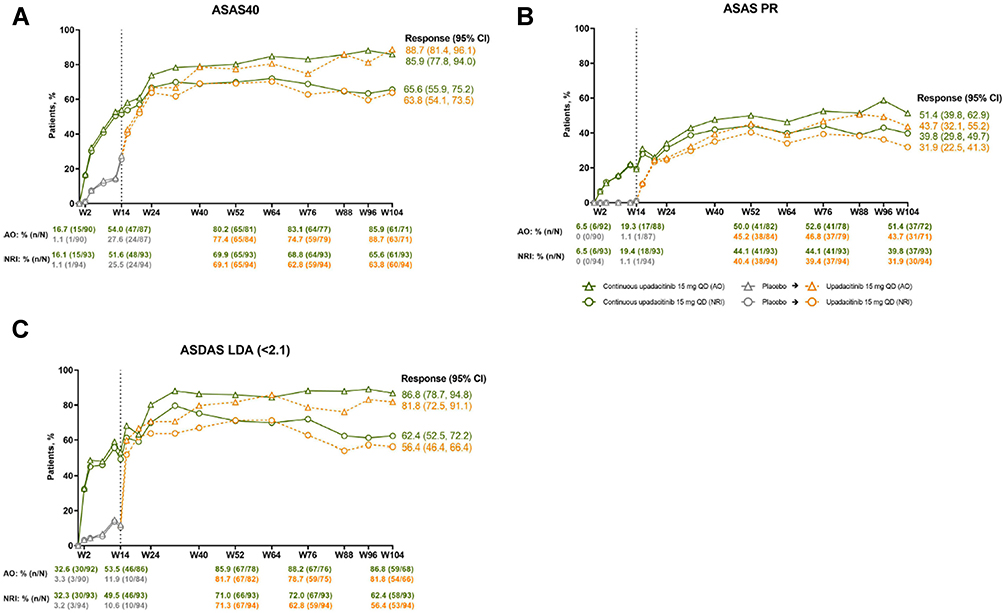
ASAS-HI improvement ≥30%, ASDAS LDA status and ASAS40 response
Por um escritor misterioso
Descrição
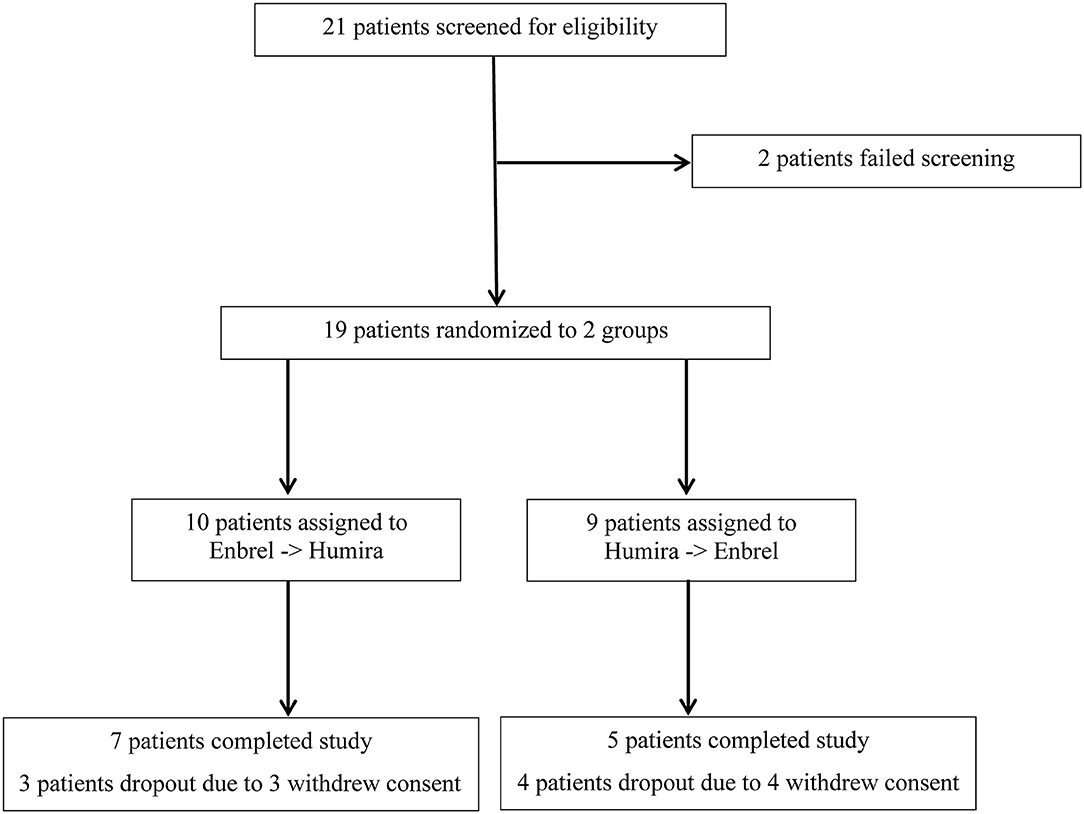
Frontiers Head-to-Head Comparison of Etanercept vs. Adalimumab in the Treatment of Ankylosing Spondylitis: An Open-Label Randomized Controlled Crossover Clinical Trial

Full article: Effectiveness of bDMARDs in ankylosing spondylitis patients by biologic use: experience from the CorEvitas PsA/SpA Registry

Effect of body mass index on treatment response of biologic/targeted-synthetic DMARDs in patients with rheumatoid arthritis, psoriatic arthritis or axial spondyloarthritis. A systematic review - ScienceDirect

Disease Control Data, Ankylosing Spondylitis
Management of axial spondyloarthritis

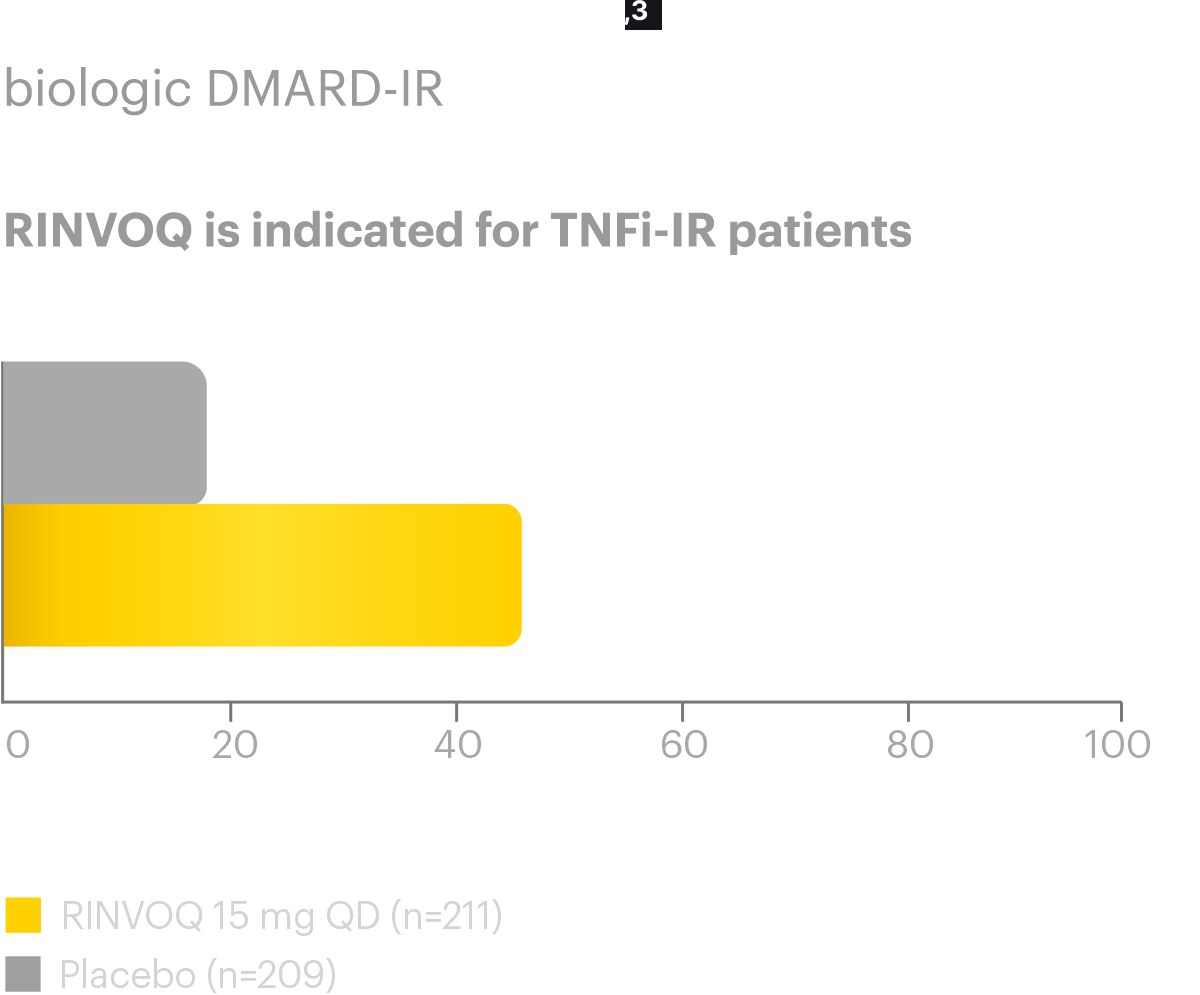
Assessment of SpondyloArthritis international Society criteria for 20%
Disease Control Data, Ankylosing Spondylitis

Oral Abstracts - 2020 - International Journal of Rheumatic Diseases - Wiley Online Library
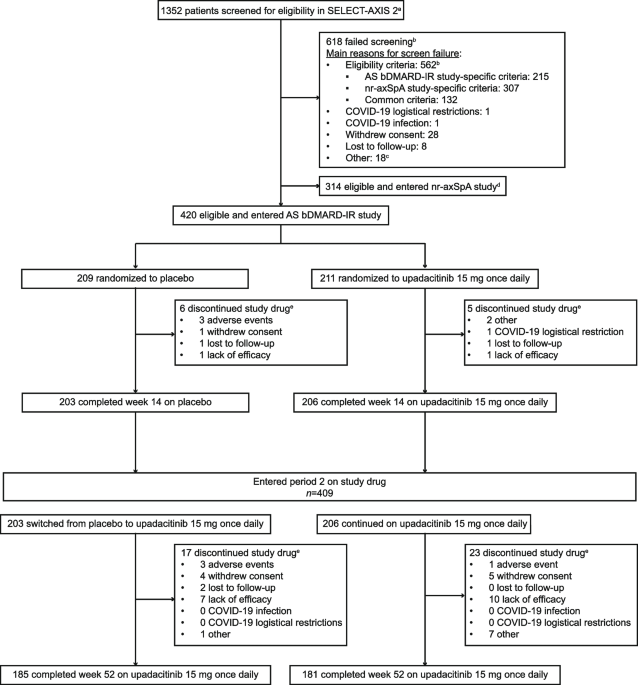
Efficacy and safety of upadacitinib in patients with ankylosing spondylitis refractory to biologic therapy: 1-year results from the open-label extension of a phase III study, Arthritis Research & Therapy

Percentages of patients achieving ASDAS LDA (
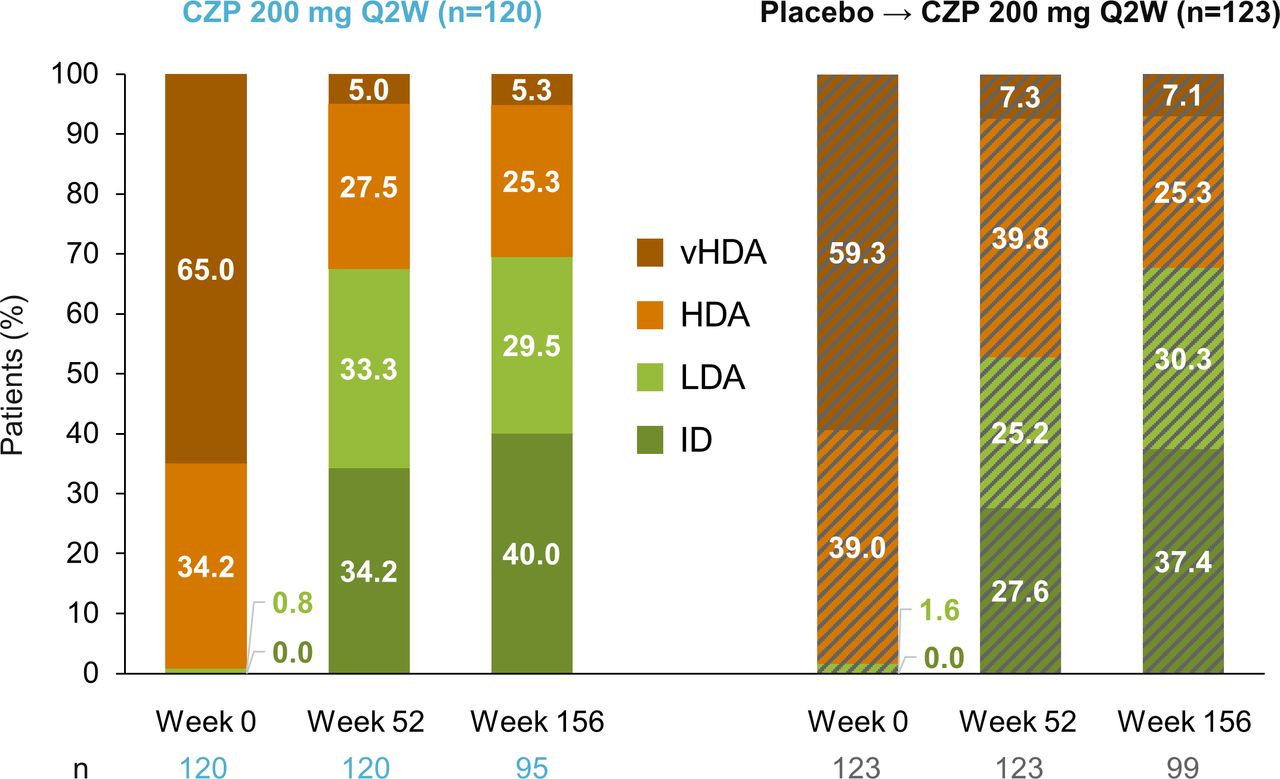
Long-term safety and clinical outcomes of certolizumab pegol treatment in patients with active non-radiographic axial spondyloarthritis: 3-year results from the phase 3 C-axSpAnd study

Disease Control Data, Ankylosing Spondylitis
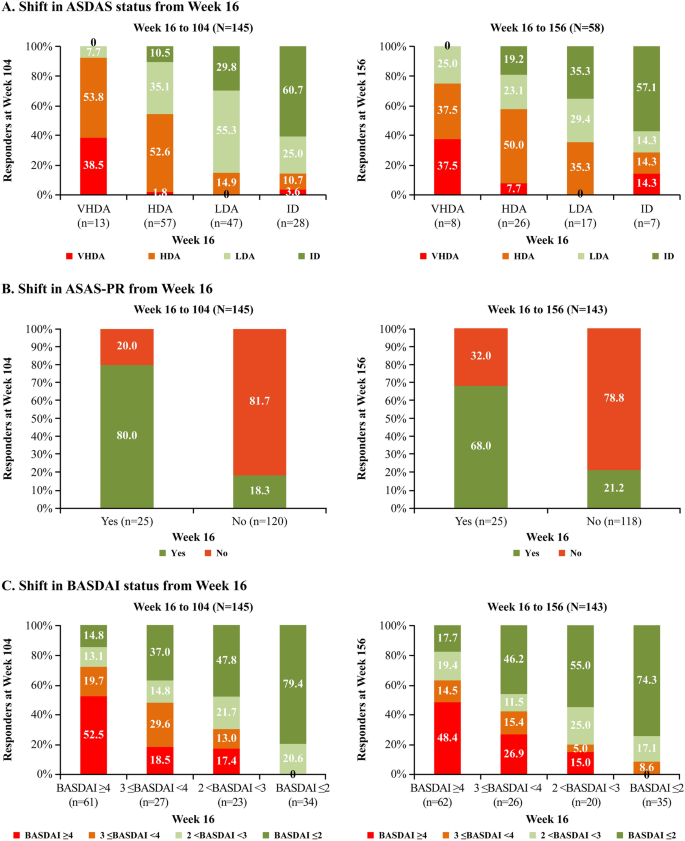
Achievement of Remission Endpoints with Secukinumab Over 3 Years in Active Ankylosing Spondylitis: Pooled Analysis of Two Phase 3 Studies
de
por adulto (o preço varia de acordo com o tamanho do grupo)







