FDA allows Houston cancer doctor to resume drug trial
Por um escritor misterioso
Descrição
Federal regulators have lifted a partial hold on a clinical trial performed by Stanislaw

Radiotherapy and paclitaxel plus pazopanib or placebo in anaplastic thyroid cancer (NRG/RTOG 0912): a randomised, double-blind, placebo-controlled, multicentre, phase 2 trial - The Lancet Oncology

The global burden of adolescent and young adult cancer in 2019: a systematic analysis for the Global Burden of Disease Study 2019 - The Lancet Oncology

Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study - The Lancet Oncology

Oncolytic DNX-2401 Virus for Pediatric Diffuse Intrinsic Pontine Glioma
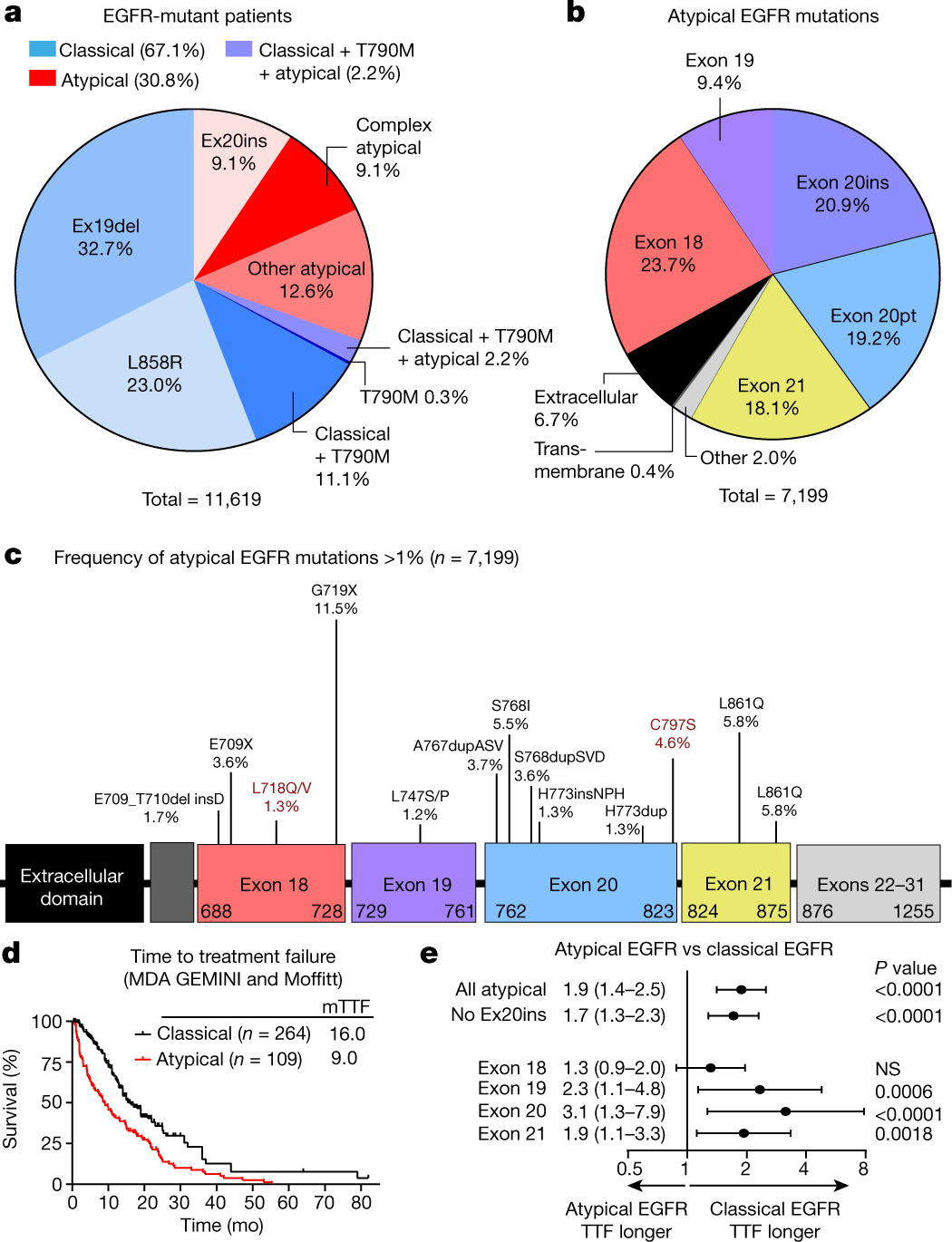
Structure-based classification predicts drug response in EGFR-mutant NSCLC

Funda Meric-Bernstam MD Anderson Cancer Center

Ashish M. Kamat MD Anderson Cancer Center
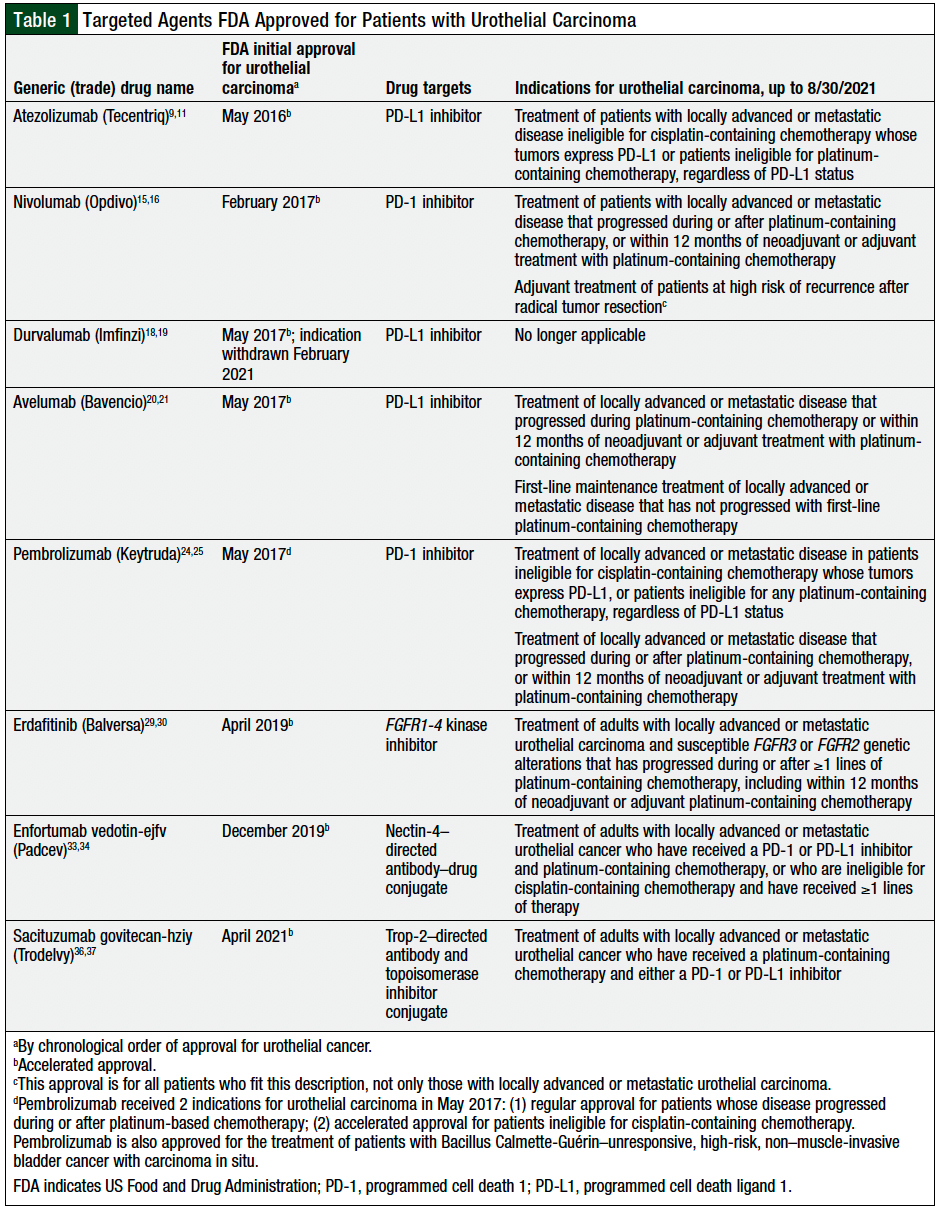
Recent Targeted Therapies Approved for the Treatment of Patients with Urothelial Carcinoma

Doctor claims to cure pediatric cancer, critics skeptical

James P. Allison, Ph.D., Immunotherapy Researcher
de
por adulto (o preço varia de acordo com o tamanho do grupo)







